Can an Enzyme Be Used Again
A fundamental chore of proteins is to act equally enzymes—catalysts that increase the rate of virtually all the chemical reactions within cells. Although RNAs are capable of catalyzing some reactions, nigh biological reactions are catalyzed by proteins. In the absence of enzymatic catalysis, most biochemical reactions are so deadening that they would non occur under the mild conditions of temperature and pressure that are compatible with life. Enzymes accelerate the rates of such reactions by well over a one thousand thousand-fold, so reactions that would take years in the absence of catalysis can occur in fractions of seconds if catalyzed by the appropriate enzyme. Cells incorporate thousands of different enzymes, and their activities determine which of the many possible chemical reactions actually take place within the cell.
The Catalytic Activeness of Enzymes
Like all other catalysts, enzymes are characterized by two fundamental backdrop. First, they increment the charge per unit of chemical reactions without themselves being consumed or permanently altered by the reaction. Second, they increase reaction rates without altering the chemical equilibrium between reactants and products.
These principles of enzymatic catalysis are illustrated in the following example, in which a molecule acted upon by an enzyme (referred to as a substrate [S]) is converted to a product (P) as the result of the reaction. In the absenteeism of the enzyme, the reaction tin can be written equally follows:
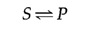
The chemical equilibrium between S and P is adamant past the laws of thermodynamics (equally discussed further in the adjacent department of this chapter) and is represented by the ratio of the forward and reverse reaction rates (Due south→P and P→S, respectively). In the presence of the appropriate enzyme, the conversion of Due south to P is accelerated, but the equilibrium between Southward and P is unaltered. Therefore, the enzyme must advance both the forward and reverse reactions as. The reaction can exist written as follows:
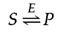
Notation that the enzyme (Due east) is not altered by the reaction, so the chemical equilibrium remains unchanged, determined solely by the thermodynamic backdrop of S and P.
The result of the enzyme on such a reaction is best illustrated by the energy changes that must occur during the conversion of S to P (Effigy two.22). The equilibrium of the reaction is determined by the terminal energy states of S and P, which are unaffected by enzymatic catalysis. In order for the reaction to go along, yet, the substrate must first be converted to a higher energy state, called the transition state. The energy required to reach the transition land (the activation free energy) constitutes a bulwark to the progress of the reaction, limiting the charge per unit of the reaction. Enzymes (and other catalysts) act past reducing the activation energy, thereby increasing the rate of reaction. The increased rate is the same in both the forwards and reverse directions, since both must pass through the same transition land.
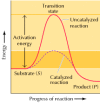
Figure ii.22
Energy diagrams for catalyzed and uncatalyzed reactions. The reaction illustrated is the simple conversion of a substrate S to a product P. Because the final energy state of P is lower than that of S, the reaction gain from left to right. For the (more...)
The catalytic activity of enzymes involves the binding of their substrates to form an enzyme-substrate complex (ES). The substrate binds to a specific region of the enzyme, chosen the active site. While bound to the active site, the substrate is converted into the production of the reaction, which is so released from the enzyme. The enzyme-catalyzed reaction tin can thus be written as follows:
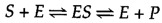
Note that Eastward appears unaltered on both sides of the equation, so the equilibrium is unaffected. Nevertheless, the enzyme provides a surface upon which the reactions converting S to P can occur more readily. This is a result of interactions between the enzyme and substrate that lower the energy of activation and favor germination of the transition state.
Mechanisms of Enzymatic Catalysis
The binding of a substrate to the active site of an enzyme is a very specific interaction. Active sites are clefts or grooves on the surface of an enzyme, commonly composed of amino acids from unlike parts of the polypeptide chain that are brought together in the 3rd structure of the folded protein. Substrates initially bind to the active site by noncovalent interactions, including hydrogen bonds, ionic bonds, and hydrophobic interactions. Once a substrate is bound to the agile site of an enzyme, multiple mechanisms tin accelerate its conversion to the product of the reaction.
Although the simple example discussed in the previous section involved only a single substrate molecule, nearly biochemical reactions involve interactions betwixt two or more dissimilar substrates. For example, the germination of a peptide bond involves the joining of 2 amino acids. For such reactions, the binding of ii or more substrates to the active site in the proper position and orientation accelerates the reaction (Figure ii.23). The enzyme provides a template upon which the reactants are brought together and properly oriented to favor the formation of the transition state in which they interact.

Figure 2.23
Enzymatic catalysis of a reaction betwixt 2 substrates. The enzyme provides a template upon which the two substrates are brought together in the proper position and orientation to react with each other.
Enzymes accelerate reactions also by altering the conformation of their substrates to approach that of the transition state. The simplest model of enzyme-substrate interaction is the lock-and-fundamental model, in which the substrate fits precisely into the active site (Effigy 2.24). In many cases, notwithstanding, the configurations of both the enzyme and substrate are modified by substrate binding—a procedure called induced fit. In such cases the conformation of the substrate is altered so that information technology more than closely resembles that of the transition country. The stress produced past such baloney of the substrate can further facilitate its conversion to the transition state past weakening critical bonds. Moreover, the transition country is stabilized by its tight binding to the enzyme, thereby lowering the required free energy of activation.

Figure two.24
Models of enzyme-substrate interaction. (A) In the lock-and-central model, the substrate fits precisely into the active site of the enzyme. (B) In the induced-fit model, substrate bounden distorts the conformations of both substrate and enzyme. This distortion (more than...)
In addition to bringing multiple substrates together and distorting the conformation of substrates to arroyo the transition state, many enzymes participate directly in the catalytic procedure. In such cases, specific amino acid side chains in the agile site may react with the substrate and form bonds with reaction intermediates. The acidic and basic amino acids are often involved in these catalytic mechanisms, as illustrated in the post-obit word of chymotrypsin every bit an example of enzymatic catalysis.
Chymotrypsin is a member of a family of enzymes (serine proteases) that assimilate proteins by catalyzing the hydrolysis of peptide bonds. The reaction can exist written every bit follows:

The dissimilar members of the serine protease family (including chymotrypsin, trypsin, elastase, and thrombin) have distinct substrate specificities; they preferentially carve peptide bonds next to different amino acids. For instance, whereas chymotrypsin digests bonds adjacent to hydrophobic amino acids, such as tryptophan and phenylalanine, trypsin digests bonds next to basic amino acids, such as lysine and arginine. All the serine proteases, nevertheless, are similar in structure and utilise the same mechanism of catalysis. The active sites of these enzymes comprise three critical amino acids—serine, histidine, and aspartate—that bulldoze hydrolysis of the peptide bond. Indeed, these enzymes are called serine proteases because of the central role of the serine residual.
Substrates bind to the serine proteases by insertion of the amino acid adjacent to the cleavage site into a pocket at the active site of the enzyme (Figure 2.25). The nature of this pocket determines the substrate specificity of the unlike members of the serine protease family. For case, the binding pocket of chymotrypsin contains hydrophobic amino acids that collaborate with the hydrophobic side chains of its preferred substrates. In dissimilarity, the bounden pocket of trypsin contains a negatively charged acidic amino acid (aspartate), which is able to grade an ionic bail with the lysine or arginine residues of its substrates.
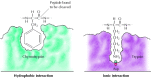
Figure ii.25
Substrate bounden past serine proteases. The amino acid adjacent to the peptide bond to exist cleaved is inserted into a pocket at the agile site of the enzyme. In chymotrypsin, the pocket binds hydrophobic amino acids; the binding pocket of trypsin contains (more...)
Substrate binding positions the peptide bond to be cleaved adjacent to the active site serine (Effigy 2.26). The proton of this serine is then transferred to the agile site histidine. The conformation of the active site favors this proton transfer because the histidine interacts with the negatively charged aspartate residue. The serine reacts with the substrate, forming a tetrahedral transition state. The peptide bond is then broken, and the C-last portion of the substrate is released from the enzyme. However, the N-terminal peptide remains spring to serine. This situation is resolved when a water molecule (the second substrate) enters the active site and reverses the preceding reactions. The proton of the water molecule is transferred to histidine, and its hydroxyl group is transferred to the peptide, forming a 2nd tetrahedral transition state. The proton is then transferred from histidine back to serine, and the peptide is released from the enzyme, completing the reaction.

Figure 2.26
Catalytic mechanism of chymotrypsin. Three amino acids at the active site (Ser-195, His-57, and Asp-102) play critical roles in catalysis.
This instance illustrates several features of enzymatic catalysis; the specificity of enzyme-substrate interactions, the positioning of unlike substrate molecules in the active site, and the involvement of active-site residues in the formation and stabilization of the transition state. Although the thousands of enzymes in cells catalyze many unlike types of chemical reactions, the same basic principles apply to their performance.
Coenzymes
In addition to bounden their substrates, the active sites of many enzymes bind other minor molecules that participate in catalysis. Prosthetic groups are pocket-size molecules jump to proteins in which they play critical functional roles. For example, the oxygen carried by myoglobin and hemoglobin is bound to heme, a prosthetic group of these proteins. In many cases metal ions (such as zinc or atomic number 26) are bound to enzymes and play primal roles in the catalytic process. In improver, various low-molecular-weight organic molecules participate in specific types of enzymatic reactions. These molecules are called coenzymes because they work together with enzymes to enhance reaction rates. In contrast to substrates, coenzymes are not irreversibly altered by the reactions in which they are involved. Rather, they are recycled and can participate in multiple enzymatic reactions.
Coenzymes serve equally carriers of several types of chemic groups. A prominent example of a coenzyme is nicotinamide adenine dinucleotide (NAD +), which functions as a carrier of electrons in oxidation-reduction reactions (Figure 2.27). NAD+ tin can accept a hydrogen ion (H+) and ii electrons (e-) from one substrate, forming NADH. NADH tin and so donate these electrons to a second substrate, re-forming NAD+. Thus, NAD+ transfers electrons from the starting time substrate (which becomes oxidized) to the 2d (which becomes reduced).

Figure ii.27
Function of NAD+ in oxidation-reduction reactions. (A) Nicotinamide adenine dinucleotide (NAD+) acts equally a carrier of electrons in oxidation-reduction reactions past accepting electrons (e-) to form NADH. (B) For example, NAD+ can accept electrons from one substrate (more...)
Several other coenzymes also act every bit electron carriers, and still others are involved in the transfer of a diverseness of additional chemical groups (east.1000., carboxyl groups and acyl groups; Tabular array 2.1). The same coenzymes role together with a variety of different enzymes to catalyze the transfer of specific chemical groups betwixt a wide range of substrates. Many coenzymes are closely related to vitamins, which contribute function or all of the structure of the coenzyme. Vitamins are not required by leaner such equally East. coli but are necessary components of the diets of man and other higher animals, which take lost the ability to synthesize these compounds.
Regulation of Enzyme Action
An of import feature of almost enzymes is that their activities are non constant but instead tin can be modulated. That is, the activities of enzymes can be regulated so that they role appropriately to encounter the varied physiological needs that may arise during the life of the cell.
One common type of enzyme regulation is feedback inhibition, in which the product of a metabolic pathway inhibits the activity of an enzyme involved in its synthesis. For instance, the amino acid isoleucine is synthesized by a series of reactions starting from the amino acid threonine (Figure two.28). The beginning step in the pathway is catalyzed by the enzyme threonine deaminase, which is inhibited by isoleucine, the end product of the pathway. Thus, an adequate amount of isoleucine in the cell inhibits threonine deaminase, blocking further synthesis of isoleucine. If the concentration of isoleucine decreases, feedback inhibition is relieved, threonine deaminase is no longer inhibited, and additional isoleucine is synthesized. Past so regulating the activeness of threonine deaminase, the prison cell synthesizes the necessary amount of isoleucine but avoids wasting energy on the synthesis of more than isoleucine than is needed.

Effigy 2.28
Feedback inhibition. The outset step in the conversion of threonine to iso-leucine is catalyzed past the enzyme threonine deaminase. The activity of this enzyme is inhibited by isoleucine, the end product of the pathway.
Feedback inhibition is i example of allosteric regulation, in which enzyme activeness is controlled by the binding of small molecules to regulatory sites on the enzyme (Figure 2.29). The term "allosteric regulation" derives from the fact that the regulatory molecules bind not to the catalytic site, but to a distinct site on the protein (allo= "other" and steric= "site"). Binding of the regulatory molecule changes the conformation of the protein, which in plow alters the shape of the agile site and the catalytic activeness of the enzyme. In the case of threonine deaminase, binding of the regulatory molecule (isoleucine) inhibits enzymatic activity. In other cases regulatory molecules serve equally activators, stimulating rather than inhibiting their target enzymes.
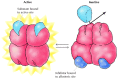
Figure ii.29
Allosteric regulation. In this example, enzyme action is inhibited by the bounden of a regulatory molecule to an allosteric site. In the absence of inhibitor, the substrate binds to the active site of the enzyme and the reaction proceeds. The binding (more than...)
The activities of enzymes tin can besides be regulated by their interactions with other proteins and by covalent modifications, such every bit the addition of phosphate groups to serine, threonine, or tyrosine residues. Phosphorylation is a particularly mutual machinery for regulating enzyme activity; the addition of phosphate groups either stimulates or inhibits the activities of many dissimilar enzymes (Effigy 2.30). For example, muscle cells respond to epinephrine (adrenaline) by breaking down glycogen into glucose, thereby providing a source of energy for increased muscular activity. The breakdown of glycogen is catalyzed by the enzyme glycogen phosphorylase, which is activated by phosphorylation in response to the bounden of epinephrine to a receptor on the surface of the muscle cell. Protein phosphorylation plays a central part in controlling not only metabolic reactions but also many other cellular functions, including cell growth and differentiation.

Effigy ii.thirty
Protein phosphorylation. Some enzymes are regulated by the addition of phosphate groups to the side-chain OH groups of serine (as shown hither), threonine, or tyrosine residues. For example, the enzyme glycogen phosphorylase, which catalyzes the conversion (more than...)
Source: https://www.ncbi.nlm.nih.gov/books/NBK9921/
0 Response to "Can an Enzyme Be Used Again"
Post a Comment